Title : Kinetics of the oxyethylation reaction of alcohols taking into account the influence of association. Apparent rate constants in the liquid phase chemical kinetics.
Abstract:
The reaction of oxyethylation of alcohols can be represented by the following scheme 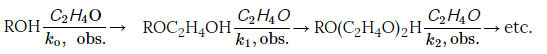
This is an irreversible alcohol-sequential reaction that can have many stages, but in this case we are mainly talking about the first stage of the reaction, characterized by the rate constant ko, obs.
Primary alcohols of normal structure C1–C7 and C10 were studied. The reaction was carried out at t = 80-180°C and P = 1.5 MPa.
Studies have shown that the reaction rate in almost all cases is of the first order in the concentration of alcohol associates. This occurs, apparently, because the reaction product remains in the associate as a terminal molecule and, having a lower reactivity, does not allow it to participate in a further reaction.
The exception is methanol. At 80 and 100 °C, the reaction rate is of the first order in alcohol concentration. This occurs because the reaction product leaves the associate. This can be explained by the fact that methanol, under these conditions, apparently exists in the form of comb-shaped associates with a coordination number close to 3, whereas other alcohols exist under these conditions in the form of linear chain associates with a coordination number close to 2. Although methanol reacts as an associate, the resulting rate constant automatically refers by default to the monomeric alcohol molecule.
In modern liquid-phase chemical kinetics, first-order reactions are very common. This is due to the fact that although an associate almost always enters into a reaction, almost as often the reaction product leaves the associate. First order in the concentration of the associated reactant is observed and the default rate constant automatically refers to the monomeric molecule of the associated reactant. The differences can be quite significant. For example, an ethanol molecule in a state close to monomeric (n≈1, solvent dodecane) at 100 °C has a rate constant for the oxyethylation reaction k o, obs. =1×10-3L2/mol2 •s. In almost pure alcohol (n=25.7) k o, obs. =11,4×10-3L2/mol2 •s. That is, the ratio of rate constants calculated based on the alcohol concentration will be equal to 11.4. If the calculation is carried out based on the concentrations of associates, then the ratio will be another 25.7 times greater, that is, it will be equal to 290.
Thus, in liquid-phase chemical kinetics, the first-order rate constants with respect to the associated component are apparent. They do not reflect the actual reactivity of monomeric molecules. Ideally, the parameters of the associate to which this constant refers should be specified.




